RNA-seq Workshop: Bioinformatics Practical (using Dask)¶
- Notebook version:
v0.0.1 - Created by:
Imperial BRC Genomics Facility - Maintained by:
Imperial BRC Genomics Facility - Docker image:
TO DO - Github repository: imperial-genomics-facility/rnaseq-notebook-image
- Created on:
2020-May-22 13:57 - Contact us: Imperial BRC Genomics Facility
- License: Apache License 2.0
In this practical session, we will investigate the basic processes required to analyse RNA-Seq experiments. Using data from the Illumina platform, we will work through quality checking (QC), read trimming, read alignment (mapping), visualization of read alignments, read counts, normalization, and differential expression and visualization using R.
Before using this notebook¶
List of prerequisites for using this example notebooks:
Supplementary Information¶
Note – some of these pages will require you to log in to view them using your normal college username/password
- File Formats:
- https://wiki.imperial.ac.uk/display/BioInfoSupport/Bioinformatics+File+Formats
- Additional information on Programs used:
- http://www.ic.ac.uk/bioinformatics-data-science-group/resources/software/next-generation-sequencing-ngs-software/
- List of software Used:
- FastQC
- MultiQC
- Trim Galore
- STAR
- featureCounts
- Samtools
- DESeq2 (R Bioconductor package)
- RSubread (R Bioconductor package)
The data for today’s tutorial was obtained from the Griffith Lab and is available using this link:
http://genomedata.org/rnaseq-tutorial/
The dataset consists of 3 replicates of a pool of tumour samples and 3 replicates of a pool of ‘normal’ samples. The libraries were prepared using the TruSeq Stranded Total RNA Sample Prep Kit and all samples were sequenced on 2 lanes of a HiSeq200 using 100bp paired end reads.
Get data¶
We are downloading fastq files from Box server.
[ ]:
%%time
!wget -q -O /tmp/practical.tar \
https://imperialcollegelondon.box.com/shared/static/9q169p5ggm52v7npkrxo5h55b6t2qttt.tar
!mkdir -p fastq_files ;cd fastq_files;tar -xf /tmp/practical.tar
Lets check the downloaded fastq files.
[2]:
ll fastq_files
total 355136
-rw-r--r-- 1 vmuser 25955505 Mar 18 2017 hcc1395_normal_rep1_r1.fastq.gz
-rw-r--r-- 1 vmuser 30766759 Mar 18 2017 hcc1395_normal_rep1_r2.fastq.gz
-rw-r--r-- 1 vmuser 25409781 Mar 18 2017 hcc1395_normal_rep2_r1.fastq.gz
-rw-r--r-- 1 vmuser 30213083 Mar 18 2017 hcc1395_normal_rep2_r2.fastq.gz
-rw-r--r-- 1 vmuser 25132378 Mar 18 2017 hcc1395_normal_rep3_r1.fastq.gz
-rw-r--r-- 1 vmuser 30174637 Mar 18 2017 hcc1395_normal_rep3_r2.fastq.gz
-rw-r--r-- 1 vmuser 30361801 Mar 18 2017 hcc1395_tumor_rep1_r1.fastq.gz
-rw-r--r-- 1 vmuser 35887220 Mar 18 2017 hcc1395_tumor_rep1_r2.fastq.gz
-rw-r--r-- 1 vmuser 29769613 Mar 18 2017 hcc1395_tumor_rep2_r1.fastq.gz
-rw-r--r-- 1 vmuser 35254974 Mar 18 2017 hcc1395_tumor_rep2_r2.fastq.gz
-rw-r--r-- 1 vmuser 29472281 Mar 18 2017 hcc1395_tumor_rep3_r1.fastq.gz
-rw-r--r-- 1 vmuser 35241854 Mar 18 2017 hcc1395_tumor_rep3_r2.fastq.gz
You can check the fastq file format for hcc1395_normal_rep1_r1.fastq.gz using the following command
[3]:
!zcat fastq_files/hcc1395_normal_rep1_r1.fastq.gz|head -4
@K00193:38:H3MYFBBXX:4:1101:10003:44458/1
TTCCTTATGAAACAGGAAGAGTCCCTGGGCCCAGGCCTGGCCCACGGTTGTCAAGGCACATCATTGCCAGCAAGCTGAAGCATACCAGCAGCCACAACCTAGATCTCATTCCCAACCCAAAGTTCTGACTTCTGTACAAACTCGTTTCCAG
+
AAFFFKKKKKKKKKKKKKKKKKKKKKKKKFKKFKKKKF<AAKKKKKKKKKKKKKKKKFKKKFKKKKKKKKKKKFKAFKKKKKKKKKKKKKKKKKKKKKKKKKKKFKKKKKKKKKKKKFKKKKKKKKKKKKFKFFKKKKKKKKKKKKFKKKK
gzip: stdout: Broken pipe
HINT: You can remind yourself of the format by looking at https://wiki.imperial.ac.uk/display/BioInfoSupport/Bioinformatics+File+Formats
Briefly: * Line 1: Sequence identifier, prefixed with ‘@’ at the beginning of the line * Line 2: Sequence * Line 3: A ‘+’ symbol delimiting the sequence from the following line. In older files this contained a second copy of the sequence identifier after the ‘+’, but this is unnecessary and results in considerable extra data having to be stored. * Line 4: The quality scores, encoded as text (ASCII characters) rather than numbers.
Fastq files are large and are commonly stored in a compressed format – created using a program called gzip. Such compressed files have a .gz on the end of their file name. Some tools can work directly on the file in its compressed form as you have seen.
Now you can count how many reads there are in this fastq file:
[4]:
!zcat fastq_files/hcc1395_normal_rep1_r1.fastq.gz| echo $((`wc -l`/4))
331958
This means read in the contents of a compressed (gzipped) fastq file line by line (zcat) and send that (| pipe symbol) to a command that counts the total number of lines in the file, divides that number by 4 ((`wc -l `/ 4)) and writes the result to the screen (echo). The back quotes tell the operating system to run the command wc -l and evaluate the result and feed that into the rest of the command – here to divide it by 4 and write the result out. Please note that forward quotes
(‘) will not have the same effect.
How many reads did you find?
Why did you divide by 4 in the command above?
Prepare sample list¶
[5]:
sample_list = [
{'sample_name':'hcc1395_normal_rep1',
'R1':'/home/vmuser/examples/fastq_files/hcc1395_normal_rep1_r1.fastq.gz',
'R2':'/home/vmuser/examples/fastq_files/hcc1395_normal_rep1_r2.fastq.gz'},
{'sample_name':'hcc1395_normal_rep2',
'R1':'/home/vmuser/examples/fastq_files/hcc1395_normal_rep2_r1.fastq.gz',
'R2':'/home/vmuser/examples/fastq_files/hcc1395_normal_rep2_r2.fastq.gz'},
{'sample_name':'hcc1395_normal_rep3',
'R1':'/home/vmuser/examples/fastq_files/hcc1395_normal_rep3_r1.fastq.gz',
'R2':'/home/vmuser/examples/fastq_files/hcc1395_normal_rep3_r2.fastq.gz'},
{'sample_name':'hcc1395_tumor_rep1',
'R1':'/home/vmuser/examples/fastq_files/hcc1395_tumor_rep1_r1.fastq.gz',
'R2':'/home/vmuser/examples/fastq_files/hcc1395_tumor_rep1_r2.fastq.gz'},
{'sample_name':'hcc1395_tumor_rep2',
'R1':'/home/vmuser/examples/fastq_files/hcc1395_tumor_rep2_r1.fastq.gz',
'R2':'/home/vmuser/examples/fastq_files/hcc1395_tumor_rep2_r2.fastq.gz'},
{'sample_name':'hcc1395_tumor_rep3',
'R1':'/home/vmuser/examples/fastq_files/hcc1395_tumor_rep3_r1.fastq.gz',
'R2':'/home/vmuser/examples/fastq_files/hcc1395_tumor_rep3_r2.fastq.gz'}
]
Create a Dask cluster¶
For this tutorial, we are using a LocalCluster with 1 worker. Feel free to replace it with more number of workers or any of the supported clusters from Dask-Jobqueue (e.g. PBS, Slurm, MOAB, SGE, LSF, and HTCondor)
[6]:
import os,subprocess
from dask.distributed import Client, LocalCluster
[7]:
cluster = LocalCluster(n_workers=1,ip='0.0.0.0',threads_per_worker=1)
Now connect to Dask cluster
[8]:
client = Client(cluster)
[9]:
client
[9]:
Client
|
Cluster
|
[10]:
if 'BINDER_LAUNCH_HOST' in os.environ:
from IPython.display import HTML
url = '<a href="{0}user/{1}/proxy/8787/status">DASK UI</a>'.\
format(
os.environ.get('JUPYTERHUB_BASE_URL'),
os.environ.get('JUPYTERHUB_CLIENT_ID').replace('jupyterhub-user-','')
)
else:
url = '<a href="http://localhost:8787/status">DASK UI</a>'
HTML(url)
[10]:
Quality Assessment¶
Now we should assess the quality of our dataset. To do this we can use a program called FastQC.
FastQC can be run interactively through a graphical interface or on the command line (by typing commands). Running the program on the command line is much faster if you are using more than one or 2 input files than going through a graphical interface. Here, we will process files from the command line and then view on a browser. All the information that the program needs to run can be specified on the command line as shown below as an example.
For clarity, we can also split this command over multiple lines (as would be done in a script as part of an analysis pipeline), using the \ (backslash) character to indicate the end of each line except the last.
fastqc \ # program name
-o outputdir \ # name of directory to put output files in (optional – default is fastq directory)
--(no)extract \ # do or do not extract the zipped output files once created -default is yes
-f fastq \ # input file format which can be set to e.g. SAM, BAM or fastq
-c contaminantfile \ # screen for a specific contaminant sequence using contents of named file (optional)
seqfile # name of input file – which can be multiple files named one after another
We will now run FastQC on all of the files in the fastq_files directory. Once this has finished and the prompt returns, we can have a look at the output html files in the fastqc_output directory by loading any of them into a browser.
For the Dask version, we are wrapping this command within a Python function for distributed tasks.
[11]:
def run_fastqc(sample_entry,fastqc_output='/home/vmuser/examples/fastqc_output'):
sample_name = sample_entry.get('sample_name')
R1_fastq = sample_entry.get('R1')
R2_fastq = sample_entry.get('R2')
cmd = ["fastqc",
"-q",
"-o {0}".format(fastqc_output),
"-t 1",
R1_fastq,
R2_fastq]
cmd = ' '.join(cmd)
subprocess.check_call(cmd, shell=True)
return sample_name
[12]:
%%time
!mkdir -p /home/vmuser/examples/fastqc_output
f = client.map(run_fastqc,sample_list)
results = client.gather(f)
CPU times: user 3.97 s, sys: 1.01 s, total: 4.98 s
Wall time: 1min 49s
[13]:
results
[13]:
['hcc1395_normal_rep1',
'hcc1395_normal_rep2',
'hcc1395_normal_rep3',
'hcc1395_tumor_rep1',
'hcc1395_tumor_rep2',
'hcc1395_tumor_rep3']
Check the status of all tasks
[14]:
[i.status for i in f]
[14]:
['finished', 'finished', 'finished', 'finished', 'finished', 'finished']
Take a look at a reports to get an idea of the quality of the data you have been given. Each section of the report is given a traffic light summary on the left pane to highlight major issues- more detail is shown in the right hand pane when you select that section, as shown in the figure above.
N.B. RNA-Seq per-base sequence content plots frequently show a warning here (orange or even red), due to bias at the start of reads – introduced by random hexamer priming/ fragmentation using transposases. This is true for most RNA-Seq libraries where a number of different kmers are enriched at the 5’ read end, affecting around the first 10-12 bases, and you often don’t need to trim or correct it. Kmer content may also show an orange warning - libraries derived from random hexamer priming almost always show sharp spikes and warnings. Some of your samples are also showing warnings on Duplicate Sequences – again a common feature of RNA-Seq libraries where over-duplication of highly expressed transcripts is tolerated in order to be able to see lowly expressed ones.
The report provides summary statistics about the numbers of reads, per-base sequence quality, per sequence GC content, sequence duplication levels and adapter content (for a detailed explanation of all the plots in the report please see the FastQC help pages). Remember, the Phred quality score represents the probability of an incorrect base call and is a measure of the reliability of each nucleotide in the read:
where Q is the Phred quality value and P the probability of error so a Phred quality score of 20 indicates a probability of error in the base call of 1 in 100 (99% accuracy). The quality values in the original fastq files are encoded in ASCII characters rather than numbers and these are interpreted by FastQC. After interpreting the full FastQC report, for other types of NGS analyses, we might want to filter or trim reads.
For RNA-seq with the current generation of alignment algorithms, read trimming may not be required, though we include it in this practical (see Del Fabbro et al 2013 - An Extensive Evaluation of Read Trimming Effects on Illumina NGS Data Analysis. PLOS ONE http://dx.doi.org/10.1371/journal.pone.0085024).
When you have seen enough, close FastQC by simply closing the tab visualising the report.
Trimming the Reads¶
In the FastQC reports for each read you can see how the quality of the reads can differ. To improve read quality they can be trimmed. Different programmes will successfully remove duplicate sequences present in the reads (adapter contamination) and can also help trim the reads if they are below a quality threshold. There are a variety of programmes for trimming reads, for this tutorial we will be using Trim Galore.
Make sure to save the trimmed reads in a new directory so that they can’t get confused with the pre-processed reads! To do this we will need to make a new directory trimmed_output.
To run Trim Galore, use a command of the following format for each pair of reads (example given for a single pair of reads):
trim_galore \ # program name
--illumina \ # trims the first 13bp of the Illumina universal adapter
-o trimmed_reads \ # specifies the output directory for the trimmed reads
--length 90 \ # length below which the trimmed reads will be discarded
-q 25 \ # minimum quality score for undiscarded reads
--paired \ # indicate that the reads are paired
s1_r1.fq.gz s1_r2.fq.gz # fastq files
Trim Galore will output a trimmed file and a trimming report for each pair of input files.
Similar like FastQC, we will use a Python function to wrap the Trim Galore run.
[15]:
def run_trim_galore(sample_entry,trimmed_output='/home/vmuser/examples/trimmed_output'):
sample_name = sample_entry.get('sample_name')
R1_fastq = sample_entry.get('R1')
R2_fastq = sample_entry.get('R2')
trim_galor_log = \
os.path.join(
trimmed_output,
'{0}_trim_galor.log'.format(sample_name))
cmd = [
"trim_galore",
"--illumina",
"--length 90",
"-q 25",
"--paired",
"-o {0}".format(trimmed_output),
R1_fastq,
R2_fastq,
"2> ",
trim_galor_log
]
cmd = ' '.join(cmd)
subprocess.check_call(cmd,shell=True)
r1_trimmed_fastq = '{0}/{1}_r1_val_1.fq.gz'.format(trimmed_output,sample_name)
r2_trimmed_fastq = '{0}/{1}_r2_val_2.fq.gz'.format(trimmed_output,sample_name)
return dict(sample_name=sample_name,R1_trimmed=r1_trimmed_fastq,R2_trimmed=r2_trimmed_fastq)
[16]:
%%time
!mkdir -p /home/vmuser/examples/trimmed_output
f = client.map(run_trim_galore,sample_list)
results = client.gather(f)
CPU times: user 18.4 s, sys: 1.89 s, total: 20.3 s
Wall time: 5min
[17]:
results
[17]:
[{'sample_name': 'hcc1395_normal_rep1',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep1_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep1_r2_val_2.fq.gz'},
{'sample_name': 'hcc1395_normal_rep2',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep2_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep2_r2_val_2.fq.gz'},
{'sample_name': 'hcc1395_normal_rep3',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep3_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep3_r2_val_2.fq.gz'},
{'sample_name': 'hcc1395_tumor_rep1',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep1_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep1_r2_val_2.fq.gz'},
{'sample_name': 'hcc1395_tumor_rep2',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep2_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep2_r2_val_2.fq.gz'},
{'sample_name': 'hcc1395_tumor_rep3',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep3_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep3_r2_val_2.fq.gz'}]
[18]:
for sample_entry in sample_list:
sample_name = sample_entry.get('sample_name')
for result in results:
trimmed_sample = result.get('sample_name')
if sample_name==trimmed_sample:
r1_trimmed_fastq = result.get('R1_trimmed')
r2_trimmed_fastq = result.get('R2_trimmed')
sample_entry.update({'R1_trimmed':r1_trimmed_fastq})
sample_entry.update({'R2_trimmed':r2_trimmed_fastq})
[19]:
sample_list
[19]:
[{'sample_name': 'hcc1395_normal_rep1',
'R1': '/home/vmuser/examples/fastq_files/hcc1395_normal_rep1_r1.fastq.gz',
'R2': '/home/vmuser/examples/fastq_files/hcc1395_normal_rep1_r2.fastq.gz',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep1_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep1_r2_val_2.fq.gz'},
{'sample_name': 'hcc1395_normal_rep2',
'R1': '/home/vmuser/examples/fastq_files/hcc1395_normal_rep2_r1.fastq.gz',
'R2': '/home/vmuser/examples/fastq_files/hcc1395_normal_rep2_r2.fastq.gz',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep2_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep2_r2_val_2.fq.gz'},
{'sample_name': 'hcc1395_normal_rep3',
'R1': '/home/vmuser/examples/fastq_files/hcc1395_normal_rep3_r1.fastq.gz',
'R2': '/home/vmuser/examples/fastq_files/hcc1395_normal_rep3_r2.fastq.gz',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep3_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep3_r2_val_2.fq.gz'},
{'sample_name': 'hcc1395_tumor_rep1',
'R1': '/home/vmuser/examples/fastq_files/hcc1395_tumor_rep1_r1.fastq.gz',
'R2': '/home/vmuser/examples/fastq_files/hcc1395_tumor_rep1_r2.fastq.gz',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep1_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep1_r2_val_2.fq.gz'},
{'sample_name': 'hcc1395_tumor_rep2',
'R1': '/home/vmuser/examples/fastq_files/hcc1395_tumor_rep2_r1.fastq.gz',
'R2': '/home/vmuser/examples/fastq_files/hcc1395_tumor_rep2_r2.fastq.gz',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep2_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep2_r2_val_2.fq.gz'},
{'sample_name': 'hcc1395_tumor_rep3',
'R1': '/home/vmuser/examples/fastq_files/hcc1395_tumor_rep3_r1.fastq.gz',
'R2': '/home/vmuser/examples/fastq_files/hcc1395_tumor_rep3_r2.fastq.gz',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep3_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep3_r2_val_2.fq.gz'}]
In order to better understand how trimming reads affects their quality, we will once again run FastQC on the data.
[20]:
%%time
!mkdir -p trimmed_fastqc
sample_name = sample_list[0].get('sample_name')
R1_fastq = sample_list[0].get('R1_trimmed')
R2_fastq = sample_list[0].get('R2_trimmed')
print('Running FastQC for sample {0}'.format(sample_name))
!fastqc \
-q \
-o trimmed_fastqc \
-t 1 \
$R1_fastq $R2_fastq
Running FastQC for sample hcc1395_normal_rep1
CPU times: user 1.54 s, sys: 297 ms, total: 1.84 s
Wall time: 17.7 s
Now open both the FastQC reports.
What are the differences between the raw and the trimmed data?
Read Alignment¶
Some words of CAUTION (for work outside of this practical session)– when using these genome indices and annotations as reference data in the later steps of analysis, it is extremely important that the SAME genome version is used throughout an analysis. Even data derived from the same human genome build (e.g. hg19) may differ in content depending on its origin. This may have a subtle or not-so-subtle effect on your analyses and is a very common point of failure; particularly when re-analysing data. Index versions are specific to the program that they were indexed for – often even down to the program VERSION number, and may simply not be recognised by other program versions. Annotations for human come in 2 main ‘flavours’ – from Ensembl or UCSC and the feature name conventions are different. This means that you need to use a genome sequence file and an annotation file from the SAME build and same origin for all steps in your analysis. If you build your BAM alignment against one genome, don’t expect to be able to view it correctly against an annotation set from a different origin…. Other differences to consider – some human genome reference sets include all the integrated endogenous retroviruses, others have had them removed, some annotations include all the non-coding RNA annotations from rFAM, others do not etc. etc. You should consider what to use, based on your biological area of interest.
For the purposes of this practical we have created an index only of chromosome 22. To create an indexed genome in STAR, a fasta file of the genome and a GTF file containing annotated transcripts are both required. Creating a genome index in star will output: binary genome sequence, suffix arrays, text chromosome names/lengths, splice junctions coordinates, and transcripts/genes information.
For this practical, we are downloading gene annotation from GENCODE and extracting the entries for chromosome 22. Also, we are fetching the chromosome 22 fasta files from UCSC genome browser.
[21]:
%%time
!mkdir -p star_ref
!wget -q -O star_ref/gencode.v34.annotation.gtf.gz \
http://ftp.ebi.ac.uk/pub/databases/gencode/Gencode_human/release_34/gencode.v34.annotation.gtf.gz
!zcat star_ref/gencode.v34.annotation.gtf.gz|grep '^chr22' > star_ref/chr22_gencode.v34.annotation.gtf
!wget -q -O star_ref/chr22.fa.gz \
http://hgdownload.soe.ucsc.edu/goldenPath/hg38/chromosomes/chr22.fa.gz
!gzip -d star_ref/chr22.fa.gz
CPU times: user 3.37 s, sys: 488 ms, total: 3.86 s
Wall time: 30.9 s
STAR
Minimum information to run STAR is of the form
STAR [options] --genomeDir REFERENCE --readFilesIn R1.fq R2.fq
Options change how the program runs and you can set them or not as required. Other input, such as the location of the input file(s) and indexed genome are obligatory.
Mapping is a computationally intensive process and would take several hours to run on a complete dataset. To allow us to illustrate the process, we have made a much smaller dataset to run against a single human chromosome. Even this will take some minutes to run.
[22]:
%%time
!STAR \
--runThreadN 1 \
--runMode genomeGenerate \
--genomeDir star_ref \
--genomeFastaFiles star_ref/chr22.fa \
--sjdbGTFfile star_ref/chr22_gencode.v34.annotation.gtf \
--sjdbOverhang 99
May 28 13:54:43 ..... started STAR run
May 28 13:54:43 ... starting to generate Genome files
WARNING: --genomeSAindexNbases 14 is too large for the genome size=50855936, which may cause seg-fault at the mapping step. Re-run genome generation with recommended --genomeSAindexNbases 11 May 28 13:54:45
May 28 13:54:45 ... starting to sort Suffix Array. This may take a long time...
May 28 13:54:45 ... sorting Suffix Array chunks and saving them to disk...
May 28 13:55:41 ... loading chunks from disk, packing SA...
May 28 13:55:42 ... finished generating suffix array
May 28 13:55:42 ... generating Suffix Array index
May 28 13:55:59 ... completed Suffix Array index
May 28 13:55:59 ..... processing annotations GTF
May 28 13:56:00 ..... inserting junctions into the genome indices
May 28 13:56:23 ... writing Genome to disk ...
May 28 13:56:23 ... writing Suffix Array to disk ...
May 28 13:56:23 ... writing SAindex to disk
May 28 13:57:12 ..... finished successfully
CPU times: user 15.8 s, sys: 2.63 s, total: 18.5 s
Wall time: 2min 29s
The stringency chosen for the mapping program plays a critical role in the analysis. Although all mapping programs have a default behaviour (i.e. what it does if you give no further instructions) this may be not be appropriate for your dataset, or for the downstream analyses you wish to undertake. Make the program too stringent and an unacceptably large percentage of your data will not be aligned and further analysed. Make the stringency too low and reads will be mapped to incorrect areas of the genome. Both cases can have a major bias on results.
Many of the parameters used above are the ENCODE standard options recommended for a long RNA-Seq analysis. You can read more about each of the options here: http://chagall.med.cornell.edu/RNASEQcourse/STARmanual.pdf
Similar to FastQC and Trim Galore, we will wrap the STAR run command within a Python function for Dask run.
[23]:
def run_star(sample_entry,
star_output='/home/vmuser/examples/star_output',
star_ref='/home/vmuser/examples/star_ref/',
star_gtf='/home/vmuser/examples/star_ref/chr22_gencode.v34.annotation.gtf'):
sample_name = sample_entry.get('sample_name')
R1_trimmed_fastq = sample_entry.get('R1_trimmed')
R2_trimmed_fastq = sample_entry.get('R2_trimmed')
star_output_prefix = os.path.join(star_output,sample_name)
cmd = [
"STAR",
"--runThreadN 1",
"--outFileNamePrefix {0}".format(star_output_prefix),
"--outSAMattributes NH HI AS NM MD",
"--runMode alignReads",
"--quantMode TranscriptomeSAM GeneCounts",
"--sjdbGTFfile {0}".format(star_gtf),
"--genomeLoad NoSharedMemory",
"--outSAMunmapped Within",
"--outSAMtype BAM SortedByCoordinate",
"--genomeDir {0}".format(star_ref),
"--outFilterMultimapNmax 20",
"--alignIntronMin 20",
"--alignSJDBoverhangMin 1",
"--outFilterMismatchNoverReadLmax 0.04",
"--alignMatesGapMax 1000000",
"--limitBAMsortRAM 12000000000",
"--outFilterMismatchNmax 99",
"--alignIntronMax 1000000",
"--alignSJoverhangMin 8",
"--readFilesCommand zcat",
"--readFilesIn {0} {1}".format(R1_trimmed_fastq, R2_trimmed_fastq)
]
cmd = ' '.join(cmd)
subprocess.check_call(cmd,shell=True)
return sample_name,"{0}Aligned.sortedByCoord.out.bam".format(star_output_prefix)
[24]:
%%time
!mkdir -p /home/vmuser/examples/star_output
f = client.map(run_star,sample_list)
results = client.gather(f)
CPU times: user 3min 16s, sys: 10.9 s, total: 3min 27s
Wall time: 15min 29s
[25]:
results
[25]:
[('hcc1395_normal_rep1',
'/home/vmuser/examples/star_output/hcc1395_normal_rep1Aligned.sortedByCoord.out.bam'),
('hcc1395_normal_rep2',
'/home/vmuser/examples/star_output/hcc1395_normal_rep2Aligned.sortedByCoord.out.bam'),
('hcc1395_normal_rep3',
'/home/vmuser/examples/star_output/hcc1395_normal_rep3Aligned.sortedByCoord.out.bam'),
('hcc1395_tumor_rep1',
'/home/vmuser/examples/star_output/hcc1395_tumor_rep1Aligned.sortedByCoord.out.bam'),
('hcc1395_tumor_rep2',
'/home/vmuser/examples/star_output/hcc1395_tumor_rep2Aligned.sortedByCoord.out.bam'),
('hcc1395_tumor_rep3',
'/home/vmuser/examples/star_output/hcc1395_tumor_rep3Aligned.sortedByCoord.out.bam')]
Check status of all the tasks
[26]:
[i.status for i in f]
[26]:
['finished', 'finished', 'finished', 'finished', 'finished', 'finished']
[27]:
for sample_entry in sample_list:
sample_name = sample_entry.get('sample_name')
for result in results:
trimmed_sample = result[0]
if sample_name==trimmed_sample:
bam = result[1]
sample_entry.update({'bam':bam})
[28]:
sample_list
[28]:
[{'sample_name': 'hcc1395_normal_rep1',
'R1': '/home/vmuser/examples/fastq_files/hcc1395_normal_rep1_r1.fastq.gz',
'R2': '/home/vmuser/examples/fastq_files/hcc1395_normal_rep1_r2.fastq.gz',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep1_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep1_r2_val_2.fq.gz',
'bam': '/home/vmuser/examples/star_output/hcc1395_normal_rep1Aligned.sortedByCoord.out.bam'},
{'sample_name': 'hcc1395_normal_rep2',
'R1': '/home/vmuser/examples/fastq_files/hcc1395_normal_rep2_r1.fastq.gz',
'R2': '/home/vmuser/examples/fastq_files/hcc1395_normal_rep2_r2.fastq.gz',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep2_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep2_r2_val_2.fq.gz',
'bam': '/home/vmuser/examples/star_output/hcc1395_normal_rep2Aligned.sortedByCoord.out.bam'},
{'sample_name': 'hcc1395_normal_rep3',
'R1': '/home/vmuser/examples/fastq_files/hcc1395_normal_rep3_r1.fastq.gz',
'R2': '/home/vmuser/examples/fastq_files/hcc1395_normal_rep3_r2.fastq.gz',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep3_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_normal_rep3_r2_val_2.fq.gz',
'bam': '/home/vmuser/examples/star_output/hcc1395_normal_rep3Aligned.sortedByCoord.out.bam'},
{'sample_name': 'hcc1395_tumor_rep1',
'R1': '/home/vmuser/examples/fastq_files/hcc1395_tumor_rep1_r1.fastq.gz',
'R2': '/home/vmuser/examples/fastq_files/hcc1395_tumor_rep1_r2.fastq.gz',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep1_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep1_r2_val_2.fq.gz',
'bam': '/home/vmuser/examples/star_output/hcc1395_tumor_rep1Aligned.sortedByCoord.out.bam'},
{'sample_name': 'hcc1395_tumor_rep2',
'R1': '/home/vmuser/examples/fastq_files/hcc1395_tumor_rep2_r1.fastq.gz',
'R2': '/home/vmuser/examples/fastq_files/hcc1395_tumor_rep2_r2.fastq.gz',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep2_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep2_r2_val_2.fq.gz',
'bam': '/home/vmuser/examples/star_output/hcc1395_tumor_rep2Aligned.sortedByCoord.out.bam'},
{'sample_name': 'hcc1395_tumor_rep3',
'R1': '/home/vmuser/examples/fastq_files/hcc1395_tumor_rep3_r1.fastq.gz',
'R2': '/home/vmuser/examples/fastq_files/hcc1395_tumor_rep3_r2.fastq.gz',
'R1_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep3_r1_val_1.fq.gz',
'R2_trimmed': '/home/vmuser/examples/trimmed_output/hcc1395_tumor_rep3_r2_val_2.fq.gz',
'bam': '/home/vmuser/examples/star_output/hcc1395_tumor_rep3Aligned.sortedByCoord.out.bam'}]
As the program runs, you will see a considerable amount of progress information streamed to the screen, reporting what the program is doing.
STAR produces multiple output files for each alignment, the key ones you should look at are; * Log files containing detailed information about the run and final mapping * A bam file containing the alignment sorted by the chromosome coordinates. * A bam file containing the alignments translated into transcript coordinates
File format information is available at https://wiki.imperial.ac.uk/display/BioInfoSupport/Bioinformatics+File+Formats NOTE: the same information in a binary BAM file takes up approximately 10 times less space than in the equivalent SAM format file – one reason why BAM files are more commonly used for storage. BAM files cannot be read unless converted to SAM files, which can be achieved using Samtools.
MultiQC report¶
A more effective way of visualising the quality of all the reads is using MultiQC. This will combine all the reports from FastQC, Trim Galore (Cutadapt) and STAR into a single, user-friendly report. To run MultiQC on the command line, navigate into the directory containing only FastQC reports simply type:
multiqc -n multiqc_out/raw_data_multiqc_report fastqc_out/.
The parameter after -n specifies the filename of the MultiQC report. As all reports automatically get named multiqc_report, once we start to look at different data this can get confusing. The full stop indicates that MultiQC should read through the current directory and compile a report of the appropriate files. This will generate an html file that can be opened the same way as the FastQC reports.
MultiQC can be run on outputs from multiple programmes, not just FastQC. More uses of MultiQC can be found by clicking the link here: https://multiqc.info/docs/#multiqc-modules
[29]:
!find fastqc_output/ -type f > multiqc_target.txt
!find trimmed_output/ -type f >> multiqc_target.txt
!find star_output/ -type f >> multiqc_target.txt
[30]:
!multiqc -q -n multiqc_report -o multiqc_output --file-list multiqc_target.txt
Searching 138 files.. [####################################] 100%
Visualising the Alignments¶
Index aligned bams¶
To create an indexed bam file it is import you use the STAR alignment output which has the extension Aligned.sortedByCoord.out.bam . (If we did not have the sorted bam files, we could also sort them using samtools).
samtools index hcc1395_normal_rep1Aligned.sortedByCoord.out.bam
Again, we will wrap this command within a Python function for Dask run.
[31]:
def run_sam_index(sample_entry):
bam_file = sample_entry.get('bam')
cmd = [
'samtools',
'index',
bam_file
]
subprocess.check_call(cmd)
return '{0}.bai'.format(bam_file)
[32]:
%%time
f = client.map(run_sam_index,sample_list)
results = client.gather(f)
CPU times: user 2.48 s, sys: 86.1 ms, total: 2.56 s
Wall time: 10.5 s
[33]:
results
[33]:
['/home/vmuser/examples/star_output/hcc1395_normal_rep1Aligned.sortedByCoord.out.bam.bai',
'/home/vmuser/examples/star_output/hcc1395_normal_rep2Aligned.sortedByCoord.out.bam.bai',
'/home/vmuser/examples/star_output/hcc1395_normal_rep3Aligned.sortedByCoord.out.bam.bai',
'/home/vmuser/examples/star_output/hcc1395_tumor_rep1Aligned.sortedByCoord.out.bam.bai',
'/home/vmuser/examples/star_output/hcc1395_tumor_rep2Aligned.sortedByCoord.out.bam.bai',
'/home/vmuser/examples/star_output/hcc1395_tumor_rep3Aligned.sortedByCoord.out.bam.bai']
This step creates a .bai Index file. This supports fast retrieval of all alignments in a specified region without having to read through the whole alignments file. These .bai files should now appear in the directory, and you should be able to see them in the left-hand panel in Jupyter, next to the terminal. In order to visualise the alignments, you need to download both the .bam and .bai files to your local PC. To do this, simply find the files and right-click, select the ‘Download’ option.
[34]:
ls star_output/*Aligned.sortedByCoord.out.bam
star_output/hcc1395_normal_rep1Aligned.sortedByCoord.out.bam
star_output/hcc1395_normal_rep2Aligned.sortedByCoord.out.bam
star_output/hcc1395_normal_rep3Aligned.sortedByCoord.out.bam
star_output/hcc1395_tumor_rep1Aligned.sortedByCoord.out.bam
star_output/hcc1395_tumor_rep2Aligned.sortedByCoord.out.bam
star_output/hcc1395_tumor_rep3Aligned.sortedByCoord.out.bam
Alignment visualization using IGV¶
It can sometimes be useful to view the alignments generated from RNA-seq and other NGS datasets in genomic context. The relevant files are in your bioinformatics_output/star_alignment directory.
We will view one of the BAM alignment files containing accepted reads using the IGV (Integrative Genomics Viewer), but first we will need to sort the file by genomic location and index it; both of which can be done using the package SAMtools. SAMtools can also be used to convert aligned output between the SAM format and its binary version BAM (but we don’t need to here), and also to sort and re-order the reads within a BAM file. Samtools can also report various statistics on the alignment the BAM contains, and allow you to manipulate the data, removing subsets to a new file etc. This is useful as BAM files store data in binary format which means that you cannot view or manipulate their contents using standard text editors (e.g. less, nano, vim). File format information is available at https://wiki.imperial.ac.uk/display/BioInfoSupport/Bioinformatics+File+Formats NOTE: the same information in a binary BAM file takes up approximately 10 times less space than in the equivalent SAM format file – one reason why BAM files are more commonly used for storage.
In a new tab in your browser open this: http://igv.org
Before you can visualise the alignments, some of the parameters need to be altered.
At the top menu bar, click the drop-down option labelled Genome and select build ‘Human (GRCh38/hg38)’; it’s important that it is the same build used during the alignments. Next in the grey bar labelled ‘IGV’, change the selection in the drop-down menu from ‘all’ to ‘chr22’.
Now it’s time to upload and visualise your data. Make sure you have downloaded the bam files from STAR with the suffix ‘Aligned.sortedByCoord.out.bam’ and their corresponding index files. In the top menu bar, click on the drop-down option labelled ‘Tracks’ and select ‘Local File..’ and upload one of the bam files containing tumour data and it’s corresponding index. Now you can navigate to a specific gene on the chromosome in order to visualise the mapped reads; in this case we will look at RBFOX2. Paste the following chromosome coordinates into the search box of the grey bar on the IGV page: chr22:35,808,893-35,811,174
This will take you to an exon on the RBFOX2 gene, were you will be able to see the alignment of reads.
Feel free to play around with the IGV settings and discover how you can visualise different details about the alignment. You can upload multiple tracks at once and compare the data.
If you are using Binder for running this tutorial, please run next two cells (after uncommenting the # from the #!http-server -s) to access an alignment view.
[35]:
if 'BINDER_LAUNCH_HOST' in os.environ:
from IPython.display import HTML
url = '<a href="{0}user/{1}/proxy/8080/bam.html">IGV</a>'.\
format(
os.environ.get('JUPYTERHUB_BASE_URL'),
os.environ.get('JUPYTERHUB_CLIENT_ID').replace('jupyterhub-user-','')
)
else:
url = '<a href="http://localhost:8080/bam.html">IGV</a>'
HTML(url)
[35]:
[36]:
#!http-server -s
When you are done, feel free to stop the above cell by clicking on the stop button from the tab menu.
Feature Counts¶
Once reads have been mapped, we can proceed to quantifying the reads, counting the reads using FeatureCounts which is a simple and fast standalone tool using a BAM file and matched genome annotation to count the number of reads which overlap annotated genes and other genomic features.
FeatureCounts works with a SAM or BAM file. You will also need the corresponding .gtf annotation file. We have provided you with a copy of the gene annotations that corresponds with the genome version used to make these bam files. Now, you can take a look at the first few lines of the gtf file containing the annotations:
[37]:
!cat star_ref/chr22_gencode.v34.annotation.gtf |head -1
chr22 ENSEMBL gene 10736171 10736283 . - . gene_id "ENSG00000277248.1"; gene_type "snRNA"; gene_name "AC145613.1"; level 3;
cat: write error: Broken pipe
In this file, you will find formatted information about each exon of each annotated gene, including strand and coordinates, exon identifier, number of exon in the gene transcript, transcript identifier, as well as information about the gene – biotype e.g. pseudogene, lincRNA and source of the annotation e.g. Havana (more information can be found in the GENCODE FAQ, e.g. https://www.gencodegenes.org/pages/data_format.html).
We will now summarize the reads mapped in each sorted BAM file at the gene level and save the results together in a file with their gene names and read counts for our sample. For this practical we will run FeatureCounts from within ‘R’ using a package called Rsubread. R is a programming language and interactive environment for statistical computing for which many specialised packages are available, including a large number written specifically to analyse NGS data. Several development environments with graphical user interfaces, including RStudio and Jupyter notebooks, can be used with R, but in this practical we will be using the most basic R environment, which is included with every R installation. Like the bash shell, it provides a command-line prompt and a history of previous commands accessed using the up and down arrow keys.
For this tutorial, we are using rpy2 to run R codes from Python interface.
[38]:
import rpy2.rinterface_lib.callbacks
import logging
[39]:
rpy2.rinterface_lib.callbacks.logger.setLevel(logging.ERROR)
[40]:
%load_ext rpy2.ipython
Now load the necessary R packages that will be used throughout this practical (these will have already been installed into R’s package library for you).
[41]:
%%R
library(DESeq2)
library(RColorBrewer)
library(gplots)
library(Rsubread)
Before we continue with analysis, we need to load our metadata file into R. This file is required by DESeq2 in order to perform differential expression analysis. We have created this file for you and you can locate the file metadata.txt
[42]:
%%file metadata.txt
Sample Group
hcc1395_normal_rep1 Normal
hcc1395_normal_rep2 Normal
hcc1395_normal_rep3 Normal
hcc1395_tumor_rep1 Tumor
hcc1395_tumor_rep2 Tumor
hcc1395_tumor_rep3 Tumor
Writing metadata.txt
[43]:
%%R
metadata <- read.table(file.path("metadata.txt"),header=T)
[44]:
%%R
head(metadata)
Sample Group
1 hcc1395_normal_rep1 Normal
2 hcc1395_normal_rep2 Normal
3 hcc1395_normal_rep3 Normal
4 hcc1395_tumor_rep1 Tumor
5 hcc1395_tumor_rep2 Tumor
6 hcc1395_tumor_rep3 Tumor
Now we fill create a list of all the files that will be given to FeatureCounts as inputs.
[45]:
%%R
files <- file.path("star_output",paste0(metadata$Sample,"Aligned.sortedByCoord.out.bam"))
[46]:
%%R
file.exists(files)
[1] TRUE TRUE TRUE TRUE TRUE TRUE
[47]:
%%R
fc<-featureCounts(files=files,annot.ext="star_ref/chr22_gencode.v34.annotation.gtf",isGTFAnnotationFile=TRUE,isPairedEnd=TRUE)
========== _____ _ _ ____ _____ ______ _____
===== / ____| | | | _ \| __ \| ____| /\ | __ \
===== | (___ | | | | |_) | |__) | |__ / \ | | | |
==== \___ \| | | | _ <| _ /| __| / /\ \ | | | |
==== ____) | |__| | |_) | | \ \| |____ / ____ \| |__| |
========== |_____/ \____/|____/|_| \_\______/_/ \_\_____/
Rsubread 2.0.0
//========================== featureCounts setting ===========================\\
|| ||
|| Input files : 6 BAM files ||
|| o hcc1395_normal_rep1Aligned.sortedByCoord.o ... ||
|| o hcc1395_normal_rep2Aligned.sortedByCoord.o ... ||
|| o hcc1395_normal_rep3Aligned.sortedByCoord.o ... ||
|| o hcc1395_tumor_rep1Aligned.sortedByCoord.ou ... ||
|| o hcc1395_tumor_rep2Aligned.sortedByCoord.ou ... ||
|| o hcc1395_tumor_rep3Aligned.sortedByCoord.ou ... ||
|| ||
|| Annotation : chr22_gencode.v34.annotation.gtf (GTF) ||
|| Dir for temp files : . ||
|| Threads : 1 ||
|| Level : meta-feature level ||
|| Paired-end : yes ||
|| Multimapping reads : counted ||
|| Multi-overlapping reads : not counted ||
|| Min overlapping bases : 1 ||
|| ||
|| Chimeric reads : counted ||
|| Both ends mapped : not required ||
|| ||
\\============================================================================//
//================================= Running ==================================\\
|| ||
|| Load annotation file chr22_gencode.v34.annotation.gtf ... ||
|| Features : 29779 ||
|| Meta-features : 1388 ||
|| Chromosomes/contigs : 1 ||
|| ||
|| Process BAM file hcc1395_normal_rep1Aligned.sortedByCoord.out.bam... ||
|| Paired-end reads are included. ||
|| Total alignments : 325597 ||
|| Successfully assigned alignments : 237099 (72.8%) ||
|| Running time : 0.03 minutes ||
|| ||
|| Process BAM file hcc1395_normal_rep2Aligned.sortedByCoord.out.bam... ||
|| Paired-end reads are included. ||
|| Total alignments : 327335 ||
|| Successfully assigned alignments : 239064 (73.0%) ||
|| Running time : 0.03 minutes ||
|| ||
|| Process BAM file hcc1395_normal_rep3Aligned.sortedByCoord.out.bam... ||
|| Paired-end reads are included. ||
|| Total alignments : 327039 ||
|| Successfully assigned alignments : 238773 (73.0%) ||
|| Running time : 0.03 minutes ||
|| ||
|| Process BAM file hcc1395_tumor_rep1Aligned.sortedByCoord.out.bam... ||
|| Paired-end reads are included. ||
|| Total alignments : 383344 ||
|| Successfully assigned alignments : 295165 (77.0%) ||
|| Running time : 0.03 minutes ||
|| ||
|| Process BAM file hcc1395_tumor_rep2Aligned.sortedByCoord.out.bam... ||
|| Paired-end reads are included. ||
|| Total alignments : 385043 ||
|| Successfully assigned alignments : 296705 (77.1%) ||
|| Running time : 0.04 minutes ||
|| ||
|| Process BAM file hcc1395_tumor_rep3Aligned.sortedByCoord.out.bam... ||
|| Paired-end reads are included. ||
|| Total alignments : 384465 ||
|| Successfully assigned alignments : 295584 (76.9%) ||
|| Running time : 0.03 minutes ||
|| ||
|| Write the final count table. ||
|| Write the read assignment summary. ||
|| ||
\\============================================================================//
You can visualise the counts produced by FeatureCounts by typing
[48]:
%%R
head(fc$counts)
hcc1395.normal.rep1Aligned.sortedByCoord.out.bam
ENSG00000277248.1 0
ENSG00000283047.1 0
ENSG00000279973.2 0
ENSG00000226444.2 0
ENSG00000276871.1 0
ENSG00000288262.1 1
hcc1395.normal.rep2Aligned.sortedByCoord.out.bam
ENSG00000277248.1 0
ENSG00000283047.1 0
ENSG00000279973.2 0
ENSG00000226444.2 0
ENSG00000276871.1 0
ENSG00000288262.1 1
hcc1395.normal.rep3Aligned.sortedByCoord.out.bam
ENSG00000277248.1 0
ENSG00000283047.1 0
ENSG00000279973.2 0
ENSG00000226444.2 0
ENSG00000276871.1 0
ENSG00000288262.1 0
hcc1395.tumor.rep1Aligned.sortedByCoord.out.bam
ENSG00000277248.1 0
ENSG00000283047.1 1
ENSG00000279973.2 0
ENSG00000226444.2 0
ENSG00000276871.1 0
ENSG00000288262.1 0
hcc1395.tumor.rep2Aligned.sortedByCoord.out.bam
ENSG00000277248.1 0
ENSG00000283047.1 1
ENSG00000279973.2 0
ENSG00000226444.2 0
ENSG00000276871.1 0
ENSG00000288262.1 0
hcc1395.tumor.rep3Aligned.sortedByCoord.out.bam
ENSG00000277248.1 0
ENSG00000283047.1 0
ENSG00000279973.2 0
ENSG00000226444.2 0
ENSG00000276871.1 0
ENSG00000288262.1 0
Downstream Analysis in R¶
In this practical, we will focus on DESeq25, which is suitable for most subsequent analyses.
Now that you have successfully created the counts using FeatureCounts, you can load the data into a DESeq2 data set which is needed to perform Differential Expression Analysis. First, we will need to add a 1 to each position in the data frame as downstream applications do not allow for rows to have multiple zeroes.
[49]:
%%R
fc$counts <- fc$counts+1
[50]:
%%R
dds <- DESeqDataSetFromMatrix(countData=fc$counts,colData=metadata,design= ~Group)
[51]:
%%R
dds
class: DESeqDataSet
dim: 1388 6
metadata(1): version
assays(1): counts
rownames(1388): ENSG00000277248.1 ENSG00000283047.1 ...
ENSG00000184319.16 ENSG00000079974.17
rowData names(0):
colnames(6): hcc1395.normal.rep1Aligned.sortedByCoord.out.bam
hcc1395.normal.rep2Aligned.sortedByCoord.out.bam ...
hcc1395.tumor.rep2Aligned.sortedByCoord.out.bam
hcc1395.tumor.rep3Aligned.sortedByCoord.out.bam
colData names(2): Sample Group
Let’s now estimate the size factor (library size) and run the normalization on our countMatrix saving it into a new object. The estimateSizeFactors function estimates the size factors using the “median ratio method”.
A reference for the method: http://dx.doi.org/10.1186/gb-2010-11-10-r106
[52]:
%%R
dds <- estimateSizeFactors(dds)
[53]:
%%R
normalized_counts <- counts(dds, normalized=TRUE)
[54]:
%%R
head(normalized_counts)
hcc1395.normal.rep1Aligned.sortedByCoord.out.bam
ENSG00000277248.1 1
ENSG00000283047.1 1
ENSG00000279973.2 1
ENSG00000226444.2 1
ENSG00000276871.1 1
ENSG00000288262.1 2
hcc1395.normal.rep2Aligned.sortedByCoord.out.bam
ENSG00000277248.1 1
ENSG00000283047.1 1
ENSG00000279973.2 1
ENSG00000226444.2 1
ENSG00000276871.1 1
ENSG00000288262.1 2
hcc1395.normal.rep3Aligned.sortedByCoord.out.bam
ENSG00000277248.1 1
ENSG00000283047.1 1
ENSG00000279973.2 1
ENSG00000226444.2 1
ENSG00000276871.1 1
ENSG00000288262.1 1
hcc1395.tumor.rep1Aligned.sortedByCoord.out.bam
ENSG00000277248.1 1
ENSG00000283047.1 2
ENSG00000279973.2 1
ENSG00000226444.2 1
ENSG00000276871.1 1
ENSG00000288262.1 1
hcc1395.tumor.rep2Aligned.sortedByCoord.out.bam
ENSG00000277248.1 1
ENSG00000283047.1 2
ENSG00000279973.2 1
ENSG00000226444.2 1
ENSG00000276871.1 1
ENSG00000288262.1 1
hcc1395.tumor.rep3Aligned.sortedByCoord.out.bam
ENSG00000277248.1 1
ENSG00000283047.1 1
ENSG00000279973.2 1
ENSG00000226444.2 1
ENSG00000276871.1 1
ENSG00000288262.1 1
Differential Expression Analysis¶
You have noticed that the object “metadata” indicates two possible conditions for the samples whose gene expression are stored in countData: “tumour” and “normal”. We can run a differential gene expression analysis between “tumour” and “normal” with a single command:
[55]:
%%R
dds <- DESeq(dds)
and then have a look at the results:
[56]:
%%R
res <- results(dds)
Order the results based on the adjusted p-values
[57]:
%%R
res <- res[order(res$padj),]
DESeq2 tests for differential expression by use of negative binomial generalized linear models. You can write the ordered results from the differential expression analysis to a csv file using the following command:
[58]:
%%R
write.csv(as.data.frame(res),file="Differential_Expression_Results.csv")
NOTE: this file will be saved to your current working directory in R. You can open the file on your PC (using Excel) to view the differentially expressed genes.
Filtering Low Count Genes¶
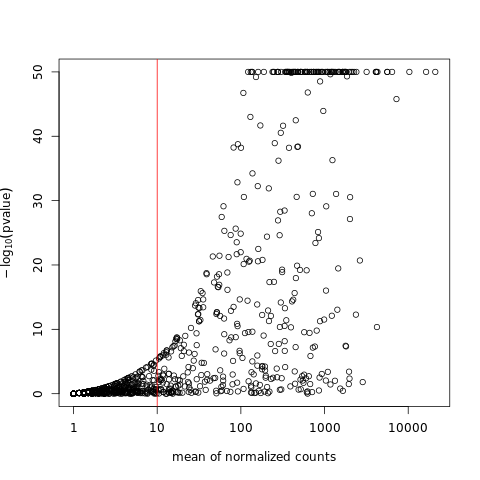
Genes with very low counts are not likely to show significant differences typically due to high dispersion. Here we visualize and cut from the results genes having a mean of normalized counts < 10.
[59]:
%%R
plot(res$baseMean, pmin(-log10(res$pvalue),50), log="x", xlab="mean of normalized counts", ylab=expression(-log[10](pvalue)))
abline(v=10,col="red",lwd=1)
[60]:
%%R
use <- res$baseMean >= 10 & !is.na(res$pvalue)
resFilt <- res[use,]
[61]:
%%R
dim(res)
[1] 1388 6
[62]:
%%R
dim(resFilt)
[1] 441 6
You can visualize the normalized counts for a single gene across the groups (e.g. the one with the lowest pvalue)
[63]:
%%R
head(resFilt)
log2 fold change (MLE): Group Tumor vs Normal
Wald test p-value: Group Tumor vs Normal
DataFrame with 6 rows and 6 columns
baseMean log2FoldChange lfcSE
<numeric> <numeric> <numeric>
ENSG00000197077.13 1145.16666666667 2.77475699225537 0.0630456555423005
ENSG00000100077.15 1517.33333333333 -1.93530481826335 0.049007958178949
ENSG00000100219.16 4157.66666666667 2.20267610268545 0.0303955907470057
ENSG00000186998.16 1024.33333333333 -3.58750735212243 0.0786042968438936
ENSG00000100234.11 4290.83333333333 9.65020991330315 0.255824251724647
ENSG00000100320.23 2402.66666666667 7.65851313277117 0.173560423699882
stat pvalue padj
<numeric> <numeric> <numeric>
ENSG00000197077.13 44.0118667715913 0 0
ENSG00000100077.15 -39.4896031211243 0 0
ENSG00000100219.16 72.466961442506 0 0
ENSG00000186998.16 -45.640092159938 0 0
ENSG00000100234.11 37.7220292769194 0 0
ENSG00000100320.23 44.1259186254013 0 0
[64]:
%%R
plotCounts(dds, gene=which.min(resFilt$padj), intgroup="Group")
Convert object “resFilt” (filtered dataset) into a dataframe (“r”) so that you can save it later as a .csv file and be able to look at it in Excel.
[65]:
%%R
r <- as.data.frame(resFilt)
[66]:
%%R
write.csv(r,"filtered_results.csv")
When you are ready (you can leave this to the end of the practical when you have finished using R in the next section) open the spread sheet in Excel and identify significantly differentially expressed genes between tumour and normal samples by applying various filters (log2fold changes, p-values, gene biotype).
Data Transformation¶
It is common practice to transform read counts to analyze these gene expression data for purposes other than differential expression, such as clustering and co-expression networks or any use in regression models where homoskedasticity (having constant variance along the range of mean values) is required. In this case DESeq2 offers a function called variance-stabilizing transformation (VST).
[67]:
%%R
newdata <- getVarianceStabilizedData(dds)
[68]:
%%R
head(newdata)
hcc1395.normal.rep1Aligned.sortedByCoord.out.bam
ENSG00000277248.1 16.37828
ENSG00000283047.1 16.37828
ENSG00000279973.2 16.37828
ENSG00000226444.2 16.37828
ENSG00000276871.1 16.37828
ENSG00000288262.1 16.38033
hcc1395.normal.rep2Aligned.sortedByCoord.out.bam
ENSG00000277248.1 16.37828
ENSG00000283047.1 16.37828
ENSG00000279973.2 16.37828
ENSG00000226444.2 16.37828
ENSG00000276871.1 16.37828
ENSG00000288262.1 16.38033
hcc1395.normal.rep3Aligned.sortedByCoord.out.bam
ENSG00000277248.1 16.37828
ENSG00000283047.1 16.37828
ENSG00000279973.2 16.37828
ENSG00000226444.2 16.37828
ENSG00000276871.1 16.37828
ENSG00000288262.1 16.37828
hcc1395.tumor.rep1Aligned.sortedByCoord.out.bam
ENSG00000277248.1 16.37828
ENSG00000283047.1 16.38033
ENSG00000279973.2 16.37828
ENSG00000226444.2 16.37828
ENSG00000276871.1 16.37828
ENSG00000288262.1 16.37828
hcc1395.tumor.rep2Aligned.sortedByCoord.out.bam
ENSG00000277248.1 16.37828
ENSG00000283047.1 16.38033
ENSG00000279973.2 16.37828
ENSG00000226444.2 16.37828
ENSG00000276871.1 16.37828
ENSG00000288262.1 16.37828
hcc1395.tumor.rep3Aligned.sortedByCoord.out.bam
ENSG00000277248.1 16.37828
ENSG00000283047.1 16.37828
ENSG00000279973.2 16.37828
ENSG00000226444.2 16.37828
ENSG00000276871.1 16.37828
ENSG00000288262.1 16.37828
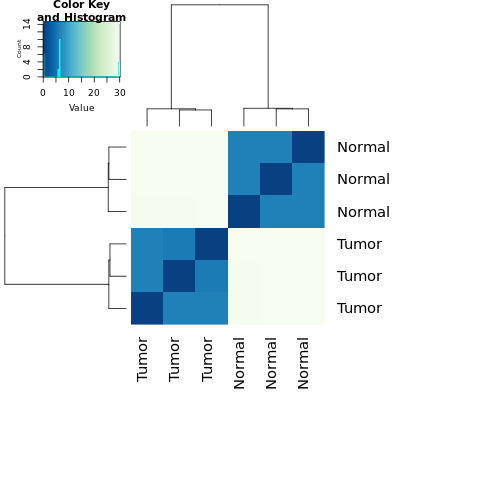
Heatmap Of Distances And Principal Component¶
We can visualize the sample-to-sample distances along with similarities and dissimilarities with a heatmap of variance-stabilized data.
[69]:
%%R
vst <- varianceStabilizingTransformation(dds, blind=TRUE)
distsRL <- dist(t(assay(vst)))
mat <- as.matrix(distsRL)
rownames(mat)<- colnames(mat)<-with(colData(dds),paste(Group))
hmcol <- colorRampPalette(brewer.pal(9, "GnBu"))(100)
heatmap.2(mat, trace="none", col = rev(hmcol), margin=c(13, 13))
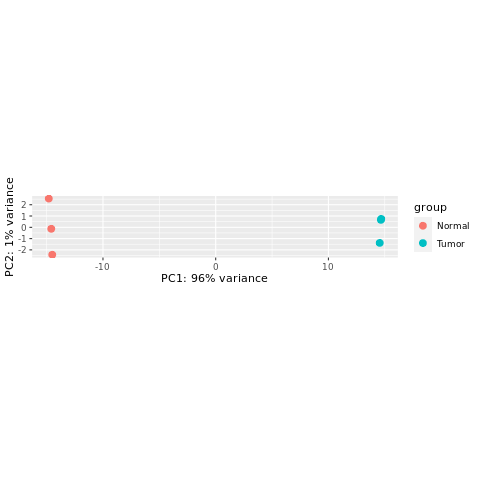
Create a PCA plot for these variance-stabilized data.
[70]:
%%R
print(plotPCA(vst, intgroup="Group"))
Now that you have finished using R, you can go back and look at the spreadsheet that we have prepared for you. If you have not already done so, copy the spreadsheet Differential_Expression_Results.csv to a PC desktop and open it using Excel.
When you have finished, don’t forget to close the browser and stop the Dask cluster.
[71]:
client.close()
cluster.close()
[ ]: